55289-561 : Flurbiprofen 100 mg Oral Tablet
| NDC: | 55289-561 |
| Labeler: | Pd-rx Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Flurbiprofen Flurbiprofen |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA074358 |
| Rev. Date: |
Appearance:
| Markings: | M;93 |
| Shapes: |
Round |
| Colors: |
 Brown Brown |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
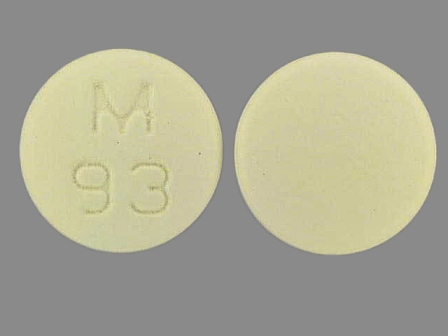
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55289-561-10: 10 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55289‑561‑10)
- 55289-561-15: 15 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55289‑561‑15)
- 55289-561-30: 30 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55289‑561‑30)
- 55289-561-60: 60 TABLET, FILM COATED IN 1 BOTTLE, PLASTIC (55289‑561‑60)
Active Ingredients:
- Flurbiprofen
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Silicon Dioxide
- Croscarmellose Sodium
- Hypromelloses
- Anhydrous Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Polydextrose
- Polyethylene Glycol
- Sodium Lauryl Sulfate
- Titanium Dioxide
- Triacetin
- Ferric Oxide Yellow
- Ferrosoferric Oxide
Pharmaceutical Classes:
- Cyclooxygenase Inhibitors [MoA]
- Nonsteroidal Anti-inflammatory Compounds [Chemical/Ingredient]
- Nonsteroidal Anti-inflammatory Drug [EPC]
Related Products:
Based on records with the same trade name.- 43063-992 Flurbiprofen 100 mg Oral Tablet, Film Coated by Pd-rx Pharmaceuticals, Inc.
- 0093-0711 Flurbiprofen 100 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0378-0076 Flurbiprofen 50 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0093 Flurbiprofen 100 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 16590-096 Flurbiprofen 100 mg Oral Tablet by Stat Rx USA LLC
- 35356-710 Flurbiprofen 100 mg Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 45865-918 Flurbiprofen 100 mg Oral Tablet, Film Coated by Medsource Pharmaceuticals
- 49999-311 Flurbiprofen 100 mg Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 50090-0509 Flurbiprofen 100 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 54569-3858 Flurbiprofen 100 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 54868-3362 Flurbiprofen 100 mg Oral Tablet by Physicians Total Care, Inc.
- 57664-164 Flurbiprofen 50 mg Oral Tablet by Caraco Pharmaceutical Laboratories, Ltd.
- 57664-165 Flurbiprofen 100 mg Oral Tablet by Caraco Pharmaceutical Laboratories, Ltd.
- 63629-1250 Flurbiprofen 100 mg Oral Tablet by Bryant Ranch Prepack
- 63629-8338 Flurbiprofen 100 mg Oral Tablet, Film Coated by Bryant Ranch Prepack
- 63629-8808 Flurbiprofen 100 mg Oral Tablet, Film Coated by Bryant Ranch Prepack
- 64950-218 Flurbiprofen 100 mg Oral Tablet by Genus Lifesciences
- 68071-2480 Flurbiprofen 100 mg Oral Tablet, Film Coated by Nucare Pharmaceuticals, Inc.
- 68071-4436 Flurbiprofen 100 mg Oral Tablet, Film Coated by Nucare Pharmaceuticals, Inc.
- 68788-7341 Flurbiprofen 100 mg Oral Tablet, Film Coated by Preferred Pharmaceuticals Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55289-560Next: 55289-562 >
Related Discussions:
flurbiprofen 100 sleeping pill
Can flurbiprofen 100 help me go to sleep? ## Hello, Ken! How are you? No, this is a nonsteroidal anti-inflammatory, it m... 1 reply
Can flurbiprofen 100 help me go to sleep? ## Hello, Ken! How are you? No, this is a nonsteroidal anti-inflammatory, it m... 1 reply
Expired topical flurbiprofen baclofen lidocaine
I have not opened it yet, but it was expired. It was prescribed on 11/27/13. Can I still use it? Is this safe? ## It doe... 1 reply
I have not opened it yet, but it was expired. It was prescribed on 11/27/13. Can I still use it? Is this safe? ## It doe... 1 reply
flurbiprofen 20% CRM #30
+GABA/, apply to affected area...
+GABA/, apply to affected area...
Flurbiprofen
...
...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.