55154-3428 : Comtan 200 mg Oral Tablet
| NDC: | 55154-3428 |
| Labeler: | Cardinal Health |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Comtan Comtan |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA020796 |
| Rev. Date: |
Appearance:
| Markings: | COMTAN |
| Shapes: |
Oval |
| Colors: |
 Orange Orange |
| Size (mm): | 17 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
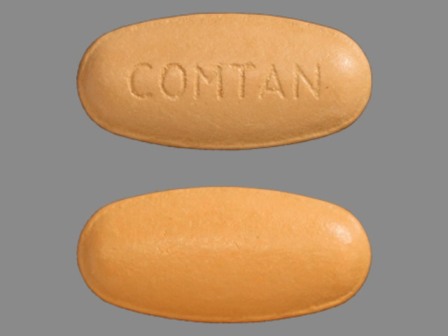
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55154-3428-0: 10 POUCH IN 1 BAG (55154‑3428‑0) > 1 TABLET, FILM COATED IN 1 POUCH
- 55154-3428-4: 100 POUCH IN 1 CARTON (55154‑3428‑4) > 1 TABLET, FILM COATED IN 1 POUCH (55154‑3428‑6)
Active Ingredients:
- Entacapone
Dosage Strength:
- 200 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Glycerin
- Ferric Oxide Red
- Hydroxypropyl Cellulose
- Magnesium Stearate
- Mannitol
- Cellulose, Microcrystalline
- Polysorbate 80
- Sucrose
- Titanium Dioxide
- Ferric Oxide Yellow
Pharmaceutical Classes:
- Catechol O-Methyltransferase Inhibitors [MoA]
- Catechol-O-Methyltransferase Inhibitor [EPC]
Related Products:
Based on records with the same trade name.- 0078-0327 Comtan 200 mg Oral Tablet by Novartis Pharmaceuticals Corporation
- 52427-800 Comtan 200 mg Oral Tablet, Film Coated by Almatica Pharma LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55154-3425Next: 55154-3429 >
Related Discussions:
Headaches & Comtan
Having daily cluster type headaches with vision loss and pain at forhead and back of neck. Noticed since starting Comtan... 2 replies
Having daily cluster type headaches with vision loss and pain at forhead and back of neck. Noticed since starting Comtan... 2 replies
carbidopa-levadopa-entacapone-
prescribed for Parkinson Disease...
prescribed for Parkinson Disease...
Comtan
Is Comtan crushable?...
Is Comtan crushable?...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.