54868-4773 : Hydroxyurea 500 mg Oral Capsule
| NDC: | 54868-4773 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Hydroxyurea Hydroxyurea |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA075143 |
| Rev. Date: |
Appearance:
| Markings: | barr;882 |
| Shapes: |
Capsule |
| Colors: |
 Purple / Purple /
 Pink Pink |
| Size (mm): | 22 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
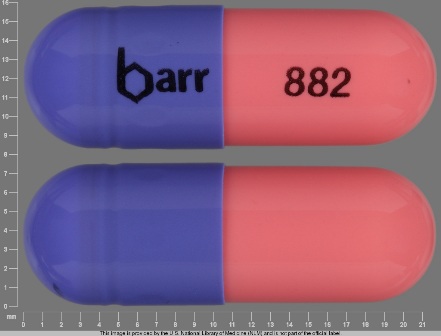
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-4773-0: 30 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑4773‑0)
- 54868-4773-1: 100 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑4773‑1)
- 54868-4773-2: 50 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑4773‑2)
- 54868-4773-3: 60 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑4773‑3)
- 54868-4773-4: 40 CAPSULE IN 1 BOTTLE, PLASTIC (54868‑4773‑4)
Active Ingredients:
- Hydroxyurea
Dosage Strength:
- 500 mg
Inactive Ingredients:
- Anhydrous Citric Acid
- Benzyl Alcohol
- Ferrosoferric Oxide
- Butylparaben
- Carboxymethylcellulose Sodium
- D&c Red No. 28
- D&c Yellow No. 10
- Aluminum Oxide
- Sodium Phosphate, Dibasic
- Edetate Calcium Disodium
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Gelatin
- Lactose Monohydrate
- Magnesium Stearate
- Methylparaben
- Shellac
- Propylparaben Sodium
- Propylene Glycol
- Ferric Oxide Red
- Sodium Lauryl Sulfate
- Sodium Propionate
- Titanium Dioxide
Pharmaceutical Classes:
- Antimetabolite [EPC]
- Urea [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0093-0882 Hydroxyurea 500 mg Oral Capsule by Teva Pharmaceuticals USA, Inc.
- 0555-0882 Hydroxyurea 500 mg Oral Capsule by Barr Laboratories Inc.
- 0904-6939 Hydroxyurea 500 mg Oral Capsule by Major Pharmaceuticals
- 10135-702 Hydroxyurea 500 mg Oral Capsule by Marlex Pharmaceuticals, Inc.
- 24535-0831 Hydroxyurea 500 mg Oral Capsule by Arab Pharmaceutical Manufacturing Co. Psc Ltd
- 42291-321 Hydroxyurea 500 mg Oral Capsule by Avkare, Inc.
- 49884-724 Hydroxyurea 500 mg Oral Capsule by Par Pharmaceutical, Inc.
- 51407-909 Hydroxyurea 500 mg Oral Capsule by Golden State Medical Supply, Inc.
- 55154-3554 Hydroxyurea 500 mg Oral Capsule by Cardinal Health 107, LLC
- 55154-7143 Hydroxyurea 500 mg Oral Capsule by Cardinal Health
- 60429-265 Hydroxyurea 500 mg Oral Capsule by Golden State Medical Supply, Inc.
- 67046-1619 Hydroxyurea 500 mg Oral Capsule by Coupler LLC
- 67184-0607 Hydroxyurea 500 mg Oral Capsule by Qilu Pharmaceutical Co., Ltd.
- 68084-284 Hydroxyurea 500 mg Oral Capsule by American Health Packaging
- 69315-164 Hydroxyurea 500 mg Oral Capsule by Leading Pharma, LLC
- 70069-820 Hydroxyurea 500 mg Oral Capsule by Somerset Therapeutics, LLC
- 70518-0916 Hydroxyurea 500 mg Oral Capsule by Remedyrepack Inc.
- 70518-3615 Hydroxyurea 500 mg Oral Capsule by Remedyrepack Inc.
- 70518-4302 Hydroxyurea 500 mg Oral Capsule by Remedyrepack Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-4770Next: 54868-4774 >
Related Discussions:
isotretinoin and hydroxyurea interaction
case of sickle cell aneamia on hydroxyurea since 4 yrs has chronic extensive acne roaccutane-(isotretinoin) has beeen re...
case of sickle cell aneamia on hydroxyurea since 4 yrs has chronic extensive acne roaccutane-(isotretinoin) has beeen re...
valium together with hydroxyurea
I take hydroxyurea daily for increased platelet production. Can't sleep at night due to stress and tension. Any risk...
I take hydroxyurea daily for increased platelet production. Can't sleep at night due to stress and tension. Any risk...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.