54569-3950 : Pyrazinamide 500 mg Oral Tablet
| NDC: | 54569-3950 |
| Labeler: | A-s Medication Solutions |
| Product Type: | Human Prescription Drug |
| Drug Name: | Pyrazinamide |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA081319 |
| Rev. Date: |
Appearance:
| Markings: | VP;012 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 13 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
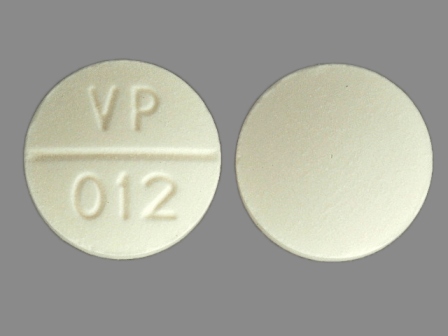
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54569-3950-0: 100 TABLET IN 1 BOTTLE (54569‑3950‑0)
Active Ingredients:
- Pyrazinamide
Dosage Strength:
- 500 mg
Inactive Ingredients:
- Silicon Dioxide
- Croscarmellose Sodium
- Calcium Phosphate, Dibasic, Dihydrate
- Cellulose, Microcrystalline
- Stearic Acid
Pharmaceutical Classes:
- Antimycobacterial [EPC]
Related Products:
Based on records with the same trade name.- 50090-0520 Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
- 50090-0521 Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
- 50090-5963 Pyrazinamide 500 mg Oral Tablet by A-s Medication Solutions
- 0054-0812 Pyrazinamide 500 mg Oral Tablet by Hikma Pharmaceuticals USA Inc
- 0904-6696 Pyrazinamide 500 mg Oral Tablet by Major Pharmaceuticals
- 10135-735 Pyrazinamide 500 mg Oral Tablet by Marlex Pharmaceuticals, Inc
- 24236-687 Pyrazinamide 500 mg Oral Tablet by Remedyrepack Inc.
- 24236-763 Pza 500 mg Oral Tablet by Remedyrepack Inc.
- 33342-447 Pyrazinamide 500 mg Oral Tablet by Macleods Pharmaceuticals Limited
- 46672-108 Pyrazinamide 500 mg Oral Tablet by Mikart, Inc.
- 49349-344 Pza 500 mg Oral Tablet by Remedyrepack Inc.
- 52125-013 Pza 500 mg Oral Tablet by Remedyrepack Inc.
- 53002-6570 Pyrazinamide 500 mg Oral Tablet by Rpk Pharmaceuticals, Inc.
- 55695-026 Pyrazinamide 500 mg Oral Tablet by Department of State Health Services, Pharmacy Branch
- 55806-012 Pza 500 mg Oral Tablet by Mikart, Inc.
- 58118-0004 Pyrazinamide 500 mg Oral Tablet by Clinical Solutions Wholesale, LLC
- 60687-138 Pyrazinamide 500 mg Oral Tablet by American Health Packaging
- 60687-789 Pyrazinamide 500 mg Oral Tablet by American Health Packaging
- 61748-012 Pza 500 mg Oral Tablet by Versapharm Incorporated
- 67253-660 Pza 500 mg Oral Tablet by Dava Pharmaceuticals, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54569-3927Next: 54569-3954 >
Related Discussions:
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Ethambutol 275mg
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Et... 4 replies
fixcom 4 Film coated tablet Antituberculosis each tablet contains Rifampicin 150 mg Isoniazid 75mg Pyrazinamide 400mg Et... 4 replies
pyrazinamide pzide 750mg
if a person if not suffering from tuberculosis suppose this pzide 750mg given to him for 16 days.What will be effect on ...
if a person if not suffering from tuberculosis suppose this pzide 750mg given to him for 16 days.What will be effect on ...
Can Pyrazinamide be used instead INH?
My doctor prescribed INH 500mg and Rifamipin 300mg. But the chemist's shop only has Pyrazinamide and says they can b...
My doctor prescribed INH 500mg and Rifamipin 300mg. But the chemist's shop only has Pyrazinamide and says they can b...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
