52125-732 : Alendronic Acid 35 mg (As Alendronate Sodium 45.7 mg) Oral Tablet
| NDC: | 52125-732 |
| Labeler: | Remedyrepack Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Alendronate Sodium Alendronate Sodium |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA075710 |
| Rev. Date: |
Appearance:
| Markings: | 93;5172 |
| Shapes: |
Square (4 sides) |
| Colors: |
 White White |
| Size (mm): | 7 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
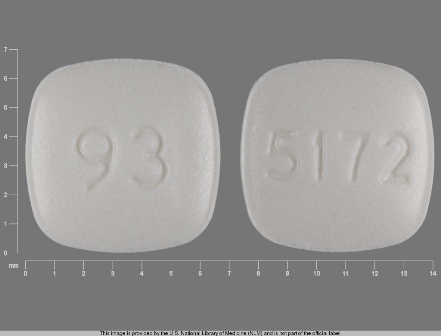
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 52125-732-17: 4 TABLET IN 1 BOX (52125‑732‑17)
Active Ingredients:
- Alendronate Sodium
Dosage Strength:
- 35 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Croscarmellose Sodium
- Magnesium Stearate
Pharmaceutical Classes:
- Bisphosphonate [EPC]
- Diphosphonates [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 52125-382 Alendronic Acid 70 mg (As Alendronate Sodium 91.4 mg) Oral Tablet by Remedyrepack Inc.
- 52125-738 Alendronic Acid 70 mg (As Alendronate Sodium 91.4 mg) Oral Tablet by Remedyrepack Inc.
- 61786-743 Alendronate Sodium 70 mg Oral Tablet by Remedyrepack Inc.
- 70518-0033 Alendronate Sodium 70 mg Oral Tablet by Remedyrepack Inc.
- 70518-0269 Alendronate Sodium 70 mg Oral Tablet by Remedyrepack Inc.
- 70518-1192 Alendronate Sodium 35 mg Oral Tablet by Remedyrepack Inc.
- 0054-0282 Alendronate Sodium 70 mg/75ml Oral Solution by Hikma Pharmaceuticals USA Inc.
- 0093-5140 Alendronic Acid 5 mg (As Alendronate Sodium 6.53 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5141 Alendronic Acid 10 mg (As Alendronate Sodium 13.1 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5142 Alendronic Acid 40 mg (As Alendronate Sodium 52.2 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5171 Alendronic Acid 70 mg (As Alendronate Sodium 91.4 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5172 Alendronic Acid 35 mg (As Alendronate Sodium 45.7 mg) Oral Tablet by Teva Pharmaceuticals USA Inc
- 0115-1676 Alendronate Sodium 5 mg Oral Tablet by Impax Generics
- 0115-1678 Alendronate Sodium 10 mg Oral Tablet by Impax Generics
- 0115-1679 Alendronate Sodium 35 mg Oral Tablet by Impax Generics
- 0115-1680 Alendronate Sodium 40 mg Oral Tablet by Impax Generics
- 0115-1681 Alendronate Sodium 70 mg Oral Tablet by Impax Generics
- 0378-3566 Alendronic Acid 5 mg (As Alendronate Sodium 6.53 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3567 Alendronic Acid 10 mg (As Alendronate Sodium 13.1 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3568 Alendronic Acid 35 mg (As Alendronate Sodium 45.7 mg) Oral Tablet by Mylan Pharmaceuticals Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 52125-731Next: 52125-734 >
Related Discussions:
Alendronate Sodium Information
What is it for? I need to know more about alendronate sodium (NDC 0093-5171-19). ## Alendronate Sodium, also called Alen... 3 replies
What is it for? I need to know more about alendronate sodium (NDC 0093-5171-19). ## Alendronate Sodium, also called Alen... 3 replies
Alendronate and muscle pain
Had muscle pain for 2 years and 10 drs didn't check the side effects of alendronate. I'm off it 7 months and sti... 4 replies
Had muscle pain for 2 years and 10 drs didn't check the side effects of alendronate. I'm off it 7 months and sti... 4 replies
Alendronate side effects
I was taking alendronate 70MG 3 month ago, but this week I got a lot of side effects such as: diarrhea, abdominal & ... 2 replies
I was taking alendronate 70MG 3 month ago, but this week I got a lot of side effects such as: diarrhea, abdominal & ... 2 replies
Alendronate Dizziness/Nausea
I have been taking Alendronate 70 mg once a week for two years, but the last month I have experienced increasingly more ... 1 reply
I have been taking Alendronate 70 mg once a week for two years, but the last month I have experienced increasingly more ... 1 reply
Alendronate generic Fosamax 70mg
Hello, been on Alendronate 70mg 4 weeks. Stopped taking it two weeks ago. Chiro told me his father fractured vertebrae. ... 1 reply
Hello, been on Alendronate 70mg 4 weeks. Stopped taking it two weeks ago. Chiro told me his father fractured vertebrae. ... 1 reply
alendronate cause positive for what drug?
Can Alendronate cause a positive result for methamphetamine? ## Hello, Troy! How are you? No, I've not found anythin... 1 reply
Can Alendronate cause a positive result for methamphetamine? ## Hello, Troy! How are you? No, I've not found anythin... 1 reply
Novo Alendronate
Bone density ## sir i am pharmacist ,own pharmacy ## This is a generic for Fosamax, which is used to treat osteoporosis ... 2 replies
Bone density ## sir i am pharmacist ,own pharmacy ## This is a generic for Fosamax, which is used to treat osteoporosis ... 2 replies
Nova Alendronate generic version?
Is there a generic version of Fosamax? ## Yes, there are several companies that have received FDA approval to produce a ... 1 reply
Is there a generic version of Fosamax? ## Yes, there are several companies that have received FDA approval to produce a ... 1 reply
Sodium Valproate and pregnancy
I have epilepsy and have taken Sodium Valproate 300 mg daily for nearly 5 years. I am now pregnant and want to know if t... 7 replies
I have epilepsy and have taken Sodium Valproate 300 mg daily for nearly 5 years. I am now pregnant and want to know if t... 7 replies
Sodium level coming down
My mother is 79yrs old. She has had an Electrolyte problem for the last 2 yrs. Sodium levels are coming down. This is th... 4 replies
My mother is 79yrs old. She has had an Electrolyte problem for the last 2 yrs. Sodium levels are coming down. This is th... 4 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.