51079-736 : Haloperidol 5 mg Oral Tablet
| NDC: | 51079-736 |
| Labeler: | Mylan Institutional Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Haloperidol Haloperidol |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA070278 |
| Rev. Date: |
Appearance:
| Markings: | MYLAN;327 |
| Shapes: |
Round |
| Colors: |
 Orange Orange |
| Size (mm): | 9 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
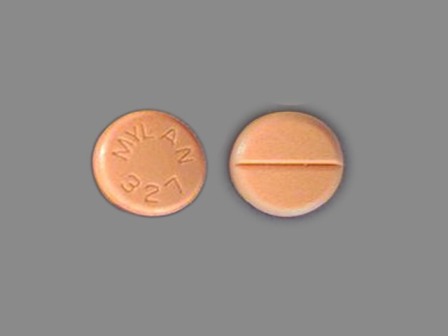
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 51079-736-20: 100 BLISTER PACK IN 1 BOX, UNIT‑DOSE (51079‑736‑20) > 1 TABLET IN 1 BLISTER PACK (51079‑736‑01)
- 51079-736-56: 10 BLISTER PACK IN 1 BOX, UNIT‑DOSE (51079‑736‑56) > 30 TABLET IN 1 BLISTER PACK (51079‑736‑30)
Active Ingredients:
- Haloperidol
Dosage Strength:
- 5 mg
Inactive Ingredients:
- Silicon Dioxide
- Fd&c Yellow No. 6
- Magnesium Stearate
- Cellulose, Microcrystalline
- Starch, Corn
- Sodium Lauryl Sulfate
Pharmaceutical Classes:
- Typical Antipsychotic [EPC]
Related Products:
Based on records with the same trade name.- 51079-431 Haloperidol 10 mg Oral Tablet by Mylan Institutional Inc.
- 51079-733 Haloperidol 0.5 mg Oral Tablet by Mylan Institutional Inc.
- 51079-734 Haloperidol 1 mg Oral Tablet by Mylan Institutional Inc.
- 51079-735 Haloperidol 2 mg Oral Tablet by Mylan Institutional Inc.
- 0069-0113 Haloperidol 5 mg/ml Intramuscular Injection, Solution by Pfizer Laboratories Div Pfizer Inc
- 0093-9604 Haloperidol 2 mg/ml Oral Solution, Concentrate by Teva Pharmaceuticals USA Inc
- 0121-0581 Haloperidol 2 mg/ml Oral Solution, Concentrate by Pharmaceutical Associates, Inc.
- 0143-9319 Haloperidol 5 mg/ml Intramuscular Injection by West-ward Pharmaceuticals Corp
- 0143-9501 Haloperidol 5 mg/ml Intramuscular Injection by West-ward Pharmaceuticals Corp
- 0143-9502 Haloperidol 5 mg/ml Intramuscular Injection by West-ward Pharmaceuticals Corp
- 0378-0214 Haloperidol 2 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0257 Haloperidol 1 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0327 Haloperidol 5 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0334 Haloperidol 10 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0335 Haloperidol 20 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-0351 Haloperidol 0.5 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0603-1290 Haloperidol 2 mg/ml Oral Solution by Qualitest Pharmaceuticals
- 0615-2594 Haloperidol 0.5 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-2595 Haloperidol 1 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-2596 Haloperidol 2 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 51079-735Next: 51079-745 >
Related Discussions:
haloperidol, cephalexin, arthrotec, oxazepam
my grandfather in in a nursing home, he is on all these meds, he is not himself anymore, we need to know what these drug... 1 reply
my grandfather in in a nursing home, he is on all these meds, he is not himself anymore, we need to know what these drug... 1 reply
Haloperidol
...
...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.