50458-657 : 24 Hr Ultram 300 mg Extended Release Tablet
| NDC: | 50458-657 |
| Labeler: | Janssen Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Ultram ER Ultram ER |
| Dosage Form: | Oral Tablet, Extended Release |
| Application #: | NDA021692 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | 300;ER |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
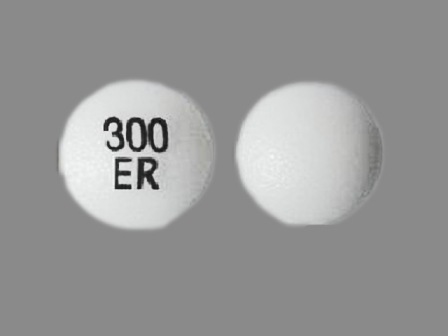
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50458-657-30: 30 TABLET, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (50458‑657‑30)
Active Ingredients:
- Tramadol Hydrochloride
Dosage Strength:
- 300 mg
Inactive Ingredients:
- Dibutyl Sebacate
- Ethylcellulose (100 Mpa.s)
- Sodium Stearyl Fumarate
- Silicon Dioxide
- Polyvinyl Alcohol
Pharmaceutical Classes:
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
Related Products:
Based on records with the same trade name.- 50458-653 24 Hr Ultram 100 mg Extended Release Tablet by Janssen Pharmaceuticals, Inc.
- 50458-655 Ultram ER 200 mg 24 Hr Extended Release Tablet by Janssen Pharmaceuticals, Inc.
- 21695-292 24 Hr Ultram 100 mg Extended Release Tablet by Rebel Distributors Corp
- 21695-563 Ultram ER 200 mg 24 Hr Extended Release Tablet by Rebel Distributors Corp
- 21695-913 24 Hr Ultram 300 mg Extended Release Tablet by Rebel Distributors Corp
- 49999-896 Ultram ER 200 mg 24 Hr Extended Release Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50458-655Next: 50458-659 >
Related Discussions:
Tramadol HCL 50 MG TAB MYLAN
one side of pill is a M and the other side of the pill is T7 ## what type of drug is tramadol hcl 50 mg. is it narcotic,... 152 replies
one side of pill is a M and the other side of the pill is T7 ## what type of drug is tramadol hcl 50 mg. is it narcotic,... 152 replies
Tramadol by Amneal vs Mylan
All our pharmacies here have switched to amneal, which doesn't do a thing except give a headache. The brand does mak... 109 replies
All our pharmacies here have switched to amneal, which doesn't do a thing except give a headache. The brand does mak... 109 replies
tramadol after August 18??
Hearing lots of places that the people who order tramadol via internet will no longer be able to. I live in a town with ... 99 replies
Hearing lots of places that the people who order tramadol via internet will no longer be able to. I live in a town with ... 99 replies
Tramadol 50 mg from Zenlabs
Hello all. I usually take Vicodin when I have bad back pain, but wanted to try something that wouldn't knock me out.... 86 replies
Hello all. I usually take Vicodin when I have bad back pain, but wanted to try something that wouldn't knock me out.... 86 replies
Tramadol classification
Is Tramadol a controlled substance, or is there any type of narcotic in them? I would really appreciate any reply. I wou... 77 replies
Is Tramadol a controlled substance, or is there any type of narcotic in them? I would really appreciate any reply. I wou... 77 replies
Tramadol Controlled Substance?
Is Tramadol considered to be a controlled medication? A narc med? ## I truly believe Tramadol is an addictive medication... 45 replies
Is Tramadol considered to be a controlled medication? A narc med? ## I truly believe Tramadol is an addictive medication... 45 replies
Tramadol withdrawal terrible stomach pain
Hello. I have searched the web and can't seem to find a problem like mine while coming off tramadol. I have horrible... 39 replies
Hello. I have searched the web and can't seem to find a problem like mine while coming off tramadol. I have horrible... 39 replies
Tramadol withdrawal side effects
My husband takes Tramadol for pain. It has caused him to be depressed. He is now addicted to the drug can't function... 31 replies
My husband takes Tramadol for pain. It has caused him to be depressed. He is now addicted to the drug can't function... 31 replies
tramadol hab pharma
I take ol-tram 100mg for chronic pain issues. From HAB pharmaceuticals. I open the capsule and tip it in my mouth, becau... 27 replies
I take ol-tram 100mg for chronic pain issues. From HAB pharmaceuticals. I open the capsule and tip it in my mouth, becau... 27 replies
Tramadol addiction
TRAMADOL=DECEPTIVE POISON. this drug is one of the nastier little secrets of the painkiller industry. from my experience... 25 replies
TRAMADOL=DECEPTIVE POISON. this drug is one of the nastier little secrets of the painkiller industry. from my experience... 25 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.