49884-869 : Dronabinol 10 mg Oral Capsule
| NDC: | 49884-869 |
| Labeler: | Par Pharmaceutical Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: | Dronabinol |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA078292 |
| Rev. Date: | |
| CSA Schedule: | CIII (US) [1] |
[1] Schedule III / IIIN Controlled Substance: Has a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence. (i.e. Products containing not more than 90 milligrams of Codeine per dosage unit [such as Tylenol with Codeine], other narcotics such as Buprenorphine (Suboxone), and Schedule IIIN non-narcotics such as Didrex, Ketamine, Phendimetrazine, and Anabolic Steroids). More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | PAR;869 |
| Shapes: |
Oval |
| Colors: |
 Orange Orange |
| Size (mm): | 12 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
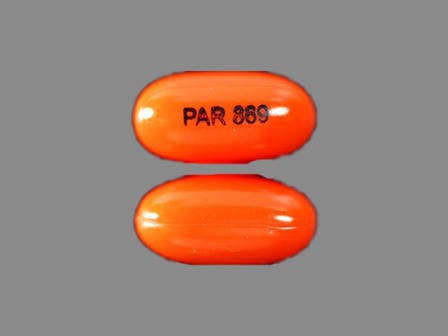
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 49884-869-01: 100 CAPSULE IN 1 BOTTLE (49884‑869‑01)
- 49884-869-02: 60 CAPSULE IN 1 BOTTLE (49884‑869‑02)
- 49884-869-05: 500 CAPSULE IN 1 BOTTLE (49884‑869‑05)
- 49884-869-15: 25 CAPSULE IN 1 BOTTLE (49884‑869‑15)
Active Ingredients:
- Dronabinol
Dosage Strength:
- 10 mg
Inactive Ingredients:
- Fd&c Yellow No. 6
- Gelatin
- Glycerin
- Water
- Sesame Oil
- Titanium Dioxide
- Ferrosoferric Oxide
- Shellac
- Isopropyl Alcohol
- Butyl Alcohol
- Propylene Glycol
- Ammonia
Pharmaceutical Classes:
- Cannabinoid [EPC]
- Cannabinoids [CS]
Related Products:
Based on records with the same trade name.- 49884-867 Dronabinol 2.5 mg Oral Capsule by Par Pharmaceutical Inc.
- 49884-868 Dronabinol 5 mg Oral Capsule by Par Pharmaceutical Inc.
- 0378-8170 Dronabinol 2.5 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-8171 Dronabinol 5 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-8172 Dronabinol 10 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0527-1450 Dronabinol 2.5 mg Oral Capsule by Lannett Company, Inc.
- 0527-1451 Dronabinol 5 mg Oral Capsule by Lannett Company, Inc.
- 0527-1452 Dronabinol 10 mg Oral Capsule by Lannett Company, Inc.
- 0527-4125 Dronabinol 2.5 mg Oral Capsule by Lannett Company, Inc.
- 0591-3591 Dronabinol 2.5 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3592 Dronabinol 5 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-3593 Dronabinol 10 mg Oral Capsule by Watson Laboratories, Inc.
- 0904-6597 Dronabinol 2.5 mg Oral Capsule by Major Pharmaceuticals
- 0904-6598 Dronabinol 5 mg Oral Capsule by Major Pharmaceuticals
- 0904-6745 Dronabinol 2.5 mg Oral Capsule by Major Pharmaceuticals
- 0904-6746 Dronabinol 5 mg Oral Capsule by Major Pharmaceuticals
- 0904-7068 Dronabinol 2.5 mg Oral Capsule by Major Pharmaceuticals
- 0904-7144 Dronabinol 2.5 mg Oral Capsule by Major Pharmaceuticals
- 0904-7145 Dronabinol 5 mg Oral Capsule by Major Pharmaceuticals
- 17478-761 Dronabinol 2.5 mg Oral Capsule by Akorn, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 49884-868Next: 49884-872 >
Related Discussions:
Dronabinol 2 5
[Federal Register: November 1, 2010 (Volume 75, Number 210)] [Page 67054-67059] Department of Justice, Drug Enforcement ...
[Federal Register: November 1, 2010 (Volume 75, Number 210)] [Page 67054-67059] Department of Justice, Drug Enforcement ...
Brown Pill Par 888=dronabinol
that is a dronabinol it is what you call a thc pill it is derived from the marijuana plant,it consist of what appearers ... 3 replies
that is a dronabinol it is what you call a thc pill it is derived from the marijuana plant,it consist of what appearers ... 3 replies
dronabinol thc
Need to know if this drug contains THC and if it's related to Marinol? ## Yes, the actual drug name is listed as Dro... 1 reply
Need to know if this drug contains THC and if it's related to Marinol? ## Yes, the actual drug name is listed as Dro... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.
