42254-039 : Rifampin 300 mg Oral Capsule
| NDC: | 42254-039 |
| Labeler: | Rebel Distributors Corp |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Rifampin Rifampin |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA065390 |
| Rev. Date: |
Appearance:
| Markings: | LANNETT;1315 |
| Shapes: |
Capsule |
| Colors: |
 Red Red |
| Size (mm): | 19 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
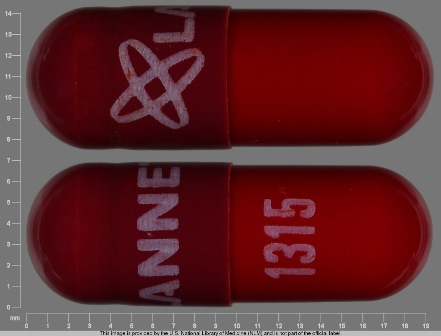
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 42254-039-30: 30 CAPSULE IN 1 BOTTLE (42254‑039‑30)
Active Ingredients:
- Rifampin
Dosage Strength:
- 300 mg
Inactive Ingredients:
- Starch, Corn
- Silicon Dioxide
- Talc
- Magnesium Stearate
- Gelatin
- Titanium Dioxide
- D&c Red No. 28
- Fd&c Red No. 40
- Fd&c Blue No. 1
- Shellac
- Alcohol
- Ferrosoferric Oxide
- Butyl Alcohol
- Propylene Glycol
- Methyl Alcohol
- Aluminum Oxide
- Fd&c Blue No. 2
- D&c Yellow No. 10
Pharmaceutical Classes:
- Rifamycin Antibacterial [EPC]
- Rifamycins [CS]
Related Products:
Based on records with the same trade name.- 21695-527 Rifampin 300 mg Oral Capsule by Rebel Distributors Corp
- 0069-0112 Rifampin 600 mg/10ml Intravenous Injection, Powder, Lyophilized, for Solution by Pfizer Laboratories Div Pfizer Inc
- 0069-0141 Rifampin 600 mg/10ml Intravenous Injection, Powder, Lyophilized, for Solution by Pfizer Laboratories Div Pfizer Inc
- 0143-9230 Rifampin 600 mg Intravenous Injection, Powder, Lyophilized, for Solution by West-ward Pharmaceuticals Corp
- 0185-0799 Rifampin 300 mg Oral Capsule by Eon Labs, Inc.
- 0185-0801 Rifampin 150 mg Oral Capsule by Eon Labs, Inc.
- 0527-1315 Rifampin 300 mg Oral Capsule by Lannett Company, Inc.
- 0527-1393 Rifampin 150 mg Oral Capsule by Lannett Company, Inc.
- 0904-5282 Rifampin 300 mg Oral Capsule by Major Pharmaceuticals
- 0904-7315 Rifampin 300 mg Oral Capsule by Major Pharmaceuticals
- 17478-151 Rifampin 600 mg/10ml Intravenous Injection, Powder, Lyophilized, for Solution by Akorn Inc.
- 17478-155 Rifampin 600 mg/10ml Intravenous Injection, Powder, Lyophilized, for Solution by Akorn
- 23155-340 Rifampin 600 mg/10ml Intravenous Injection, Powder, Lyophilized, for Solution by Heritage Pharmaceuticals Inc.
- 24236-202 Rifampin 300 mg by Remedyrepack Inc.
- 24658-801 Rifampin 150 mg Oral Capsule by Puracap Laboratories LLC Dba Blu Pharmaceuticals
- 24658-802 Rifampin 300 mg Oral Capsule by Puracap Laboratories LLC Dba Blu Pharmaceuticals
- 42806-799 Rifampin 300 mg Oral Capsule by Epic Pharma, LLC
- 42806-801 Rifampin 150 mg Oral Capsule by Epic Pharma, LLC
- 49349-250 Rifampin 300 mg Oral Capsule by Remedyrepack Inc.
- 49349-311 Rifampin 150 mg Oral Capsule by Remedyrepack Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 42254-038Next: 42254-040 >
Related Discussions:
R Cinex Rifampin And Isoniazid Capsule U P S
I have been taking R Cinex Rifampin And Isoniazid Capsule U P S since last 67 days but I missed 1day dose,which doctor p... 3 replies
I have been taking R Cinex Rifampin And Isoniazid Capsule U P S since last 67 days but I missed 1day dose,which doctor p... 3 replies
Side effects of Rifampin (rifampicin)
Hello, I would like to know if anyone is taking Rifampin and what are side effects, i read about it online but would lik... 1 reply
Hello, I would like to know if anyone is taking Rifampin and what are side effects, i read about it online but would lik... 1 reply
rifampin 150mg tablet availability
I need to locate the above mentioned medicine. I can not locate this drug in tablet form locally. Can anyone tell me abo...
I need to locate the above mentioned medicine. I can not locate this drug in tablet form locally. Can anyone tell me abo...
rifampin
infection...
infection...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.