0378-6044 : Risperidone 1 mg Disintegrating Tablet
| NDC: | 0378-6044 |
| Labeler: | Mylan Pharmaceuticals Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Risperidone Risperidone |
| Dosage Form: | Oral Tablet, Orally Disintegrating |
| Application #: | ANDA091537 |
| Rev. Date: |
Appearance:
| Markings: | M;RN1 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 10 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
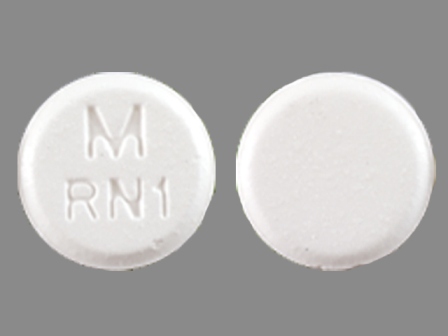
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0378-6044-01: 100 TABLET, ORALLY DISINTEGRATING IN 1 BOTTLE, PLASTIC (0378‑6044‑01)
- 0378-6044-28: 28 TABLET, ORALLY DISINTEGRATING IN 1 BOTTLE, PLASTIC (0378‑6044‑28)
- 0378-6044-93: 30 TABLET, ORALLY DISINTEGRATING IN 1 BOTTLE, PLASTIC (0378‑6044‑93)
Active Ingredients:
- Risperidone
Dosage Strength:
- 1 mg
Inactive Ingredients:
- Dimethylaminoethyl Methacrylate - Butyl Methacrylate - Methyl Methacrylate Copolymer
- Aspartame
- Crospovidone
- Magnesium Stearate
- Mannitol
- Lactose Monohydrate
- Silicon Dioxide
- Sodium Lauryl Sulfate
- Sodium Stearyl Fumarate
- Sorbitol
- Stearic Acid
Pharmaceutical Classes:
- Atypical Antipsychotic [EPC]
Related Products:
Based on records with the same trade name.- 0378-3502 Risperidone 0.25 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3505 Risperidone 0.5 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3511 Risperidone 1 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3512 Risperidone 2 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3513 Risperidone 3 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-3514 Risperidone 4 mg Oral Tablet by Mylan Pharmaceuticals Inc.
- 0378-6042 Risperidone 0.25 mg Disintegrating Tablet by Mylan Pharmaceuticals Inc.
- 0378-6043 Risperidone 0.5 mg Disintegrating Tablet by Mylan Pharmaceuticals Inc.
- 0378-6045 Risperidone 2 mg Disintegrating Tablet by Mylan Pharmaceuticals Inc.
- 0378-6046 Risperidone 3 mg Disintegrating Tablet by Mylan Pharmaceuticals Inc.
- 0378-6047 Risperidone 4 mg Disintegrating Tablet by Mylan Pharmaceuticals Inc.
- 0054-0063 Risperidone 1 mg/ml Oral Solution by Roxane Laboratories, Inc
- 0093-0221 Risperidone 0.25 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-0225 Risperidone 0.5 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7240 Risperidone 1 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7241 Risperidone 2 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7242 Risperidone 3 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-7243 Risperidone 4 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0143-9760 Risperidone 4 mg Oral Tablet by West-ward Pharmaceutical Corp
- 0143-9761 Risperidone 3 mg Oral Tablet by West-ward Pharmaceutical Corp
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0378-6043Next: 0378-6045 >
Related Discussions:
risperidone withdrawal symtoms
i would like to know the withdrawal symtoms ## Well, drawing from my husband's experience with this medication, I ca... 5 replies
i would like to know the withdrawal symtoms ## Well, drawing from my husband's experience with this medication, I ca... 5 replies
Risperidone Pill
It is orangeish pink says PATR on one side and 0.5 on the other ## Yes, this contains 0.5mgs of Risperidone, the active ... 3 replies
It is orangeish pink says PATR on one side and 0.5 on the other ## Yes, this contains 0.5mgs of Risperidone, the active ... 3 replies
Risperidone 1 Mg Better Than Seriquel 100mg
I was taken seoquel 100mg 3 times a day but got me sick an put me to abilify 30mg than my doctor took me off an put me o... 2 replies
I was taken seoquel 100mg 3 times a day but got me sick an put me to abilify 30mg than my doctor took me off an put me o... 2 replies
Risperidone withdrawel
Hi. After nearly a year of arguing with my psychiatrist and CPN about coming off risperidone Consta, I'm suddenly to... 2 replies
Hi. After nearly a year of arguing with my psychiatrist and CPN about coming off risperidone Consta, I'm suddenly to... 2 replies
risperidone 6 mg
I was on risperidone from 2002 to 2003 then quit because my dad doesn't believe I'm mental health. I have now st... 2 replies
I was on risperidone from 2002 to 2003 then quit because my dad doesn't believe I'm mental health. I have now st... 2 replies
Risperidone, Amlodipine and Sertraline drug classifications
Are any of the following medications classified as a benzodiazepine or opiate: Risperidone, Amlodipine and Sertraline? #... 1 reply
Are any of the following medications classified as a benzodiazepine or opiate: Risperidone, Amlodipine and Sertraline? #... 1 reply
Risperidone Overdose
Someone i know has taken 22 x 1mg tablets of Risperidone, an anti pychotic drug i belive... Are they in danger? How will... 1 reply
Someone i know has taken 22 x 1mg tablets of Risperidone, an anti pychotic drug i belive... Are they in danger? How will... 1 reply
risperidone 3mg
What are the common side effects for a women with Bipolar Disorder on 3mg Risperidone. ## Hello, Olivia! How are you? Th... 1 reply
What are the common side effects for a women with Bipolar Disorder on 3mg Risperidone. ## Hello, Olivia! How are you? Th... 1 reply
Risperidone gives me bad sweats anything else i could take?
Risperidone makes me sleep deep for a while but the big problem is the sweating that comes with that sleep. I have been ... 1 reply
Risperidone makes me sleep deep for a while but the big problem is the sweating that comes with that sleep. I have been ... 1 reply
Risperidone Tab 1 Mg In Place Of Risperdal
What's risperdal used for. And with purple round pill with slanted E. I was told my husband needs them by his father... 1 reply
What's risperdal used for. And with purple round pill with slanted E. I was told my husband needs them by his father... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.