0093-7306 : Celecoxib 50 mg Oral Capsule
| NDC: | 0093-7306 |
| Labeler: | Teva Pharmaceuticals USA Inc |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Celecoxib Celecoxib |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA076898 |
| Rev. Date: |
Appearance:
| Markings: | TEVA;7306 |
| Shapes: |
Capsule |
| Colors: |
 White / White /
 Orange Orange |
| Size (mm): | 14 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
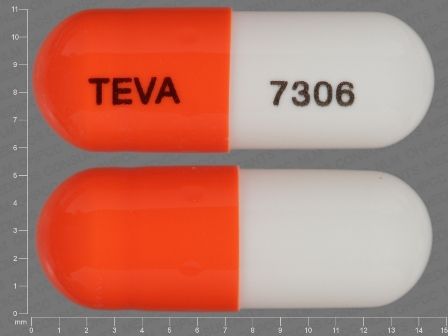
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0093-7306-06: 60 CAPSULE IN 1 BOTTLE (0093‑7306‑06)
Active Ingredients:
- Celecoxib
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Ferrosoferric Oxide
- Gelatin
- Mannitol
- Povidone K30
- Propylene Glycol
- Shellac
- Sodium Lauryl Sulfate
- Sodium Stearyl Fumarate
- Titanium Dioxide
- D&c Red No. 28
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Fd&c Red No. 40
- Potassium Hydroxide
Pharmaceutical Classes:
- Anti-Inflammatory Agents
- Non-Steroidal [CS]
- Cyclooxygenase Inhibitors [MoA]
- Nonsteroidal Anti-inflammatory Drug [EPC]
Related Products:
Based on records with the same trade name.- 0093-7165 Celecoxib 100 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7166 Celecoxib 200 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7170 Celecoxib 400 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0378-4065 Celecoxib 50 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-4066 Celecoxib 100 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-4067 Celecoxib 200 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-4068 Celecoxib 400 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-7150 Celecoxib 200 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-7160 Celecoxib 100 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-7165 Celecoxib 50 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0378-7170 Celecoxib 400 mg Oral Capsule by Mylan Pharmaceuticals Inc.
- 0591-2817 Celecoxib 50 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-2820 Celecoxib 100 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-2825 Celecoxib 200 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-2831 Celecoxib 400 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-3982 Celecoxib 50 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-3983 Celecoxib 100 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-3984 Celecoxib 200 mg Oral Capsule by Actavis Pharma, Inc.
- 0591-3985 Celecoxib 400 mg Oral Capsule by Actavis Pharma, Inc.
- 0615-8297 Celecoxib 200 mg Oral Capsule by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0093-7305Next: 0093-7314 >
Related Discussions:
Celebrex vs Celecoxib (Mylan 7150)
How close to the effects of Celebrex is Celecoxib (Mylan 7150)? So far I have not found any generics that come close to ... 2 replies
How close to the effects of Celebrex is Celecoxib (Mylan 7150)? So far I have not found any generics that come close to ... 2 replies
meloxicam 15 mg celecoxib 200 mg
can meloxicam be taken with celecoxib ? ## Hi John, According to umm.edu's drug interaction checker, there are no in... 1 reply
can meloxicam be taken with celecoxib ? ## Hi John, According to umm.edu's drug interaction checker, there are no in... 1 reply
Celecoxib 100 200mg + Diacerein 50 50mg
If any combined formulation product is available of Celecoxib 100/200mg + Diacerein 50/50mg in the Indian market, then p... 1 reply
If any combined formulation product is available of Celecoxib 100/200mg + Diacerein 50/50mg in the Indian market, then p... 1 reply
CELECOXIB
...
...
Sultamicillin and celecoxib
my question is why is my doctor gave me sultimicillin and celecoxib after my operation on thyroidectomy?...
my question is why is my doctor gave me sultimicillin and celecoxib after my operation on thyroidectomy?...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.