0085-1401 : Foradil 0.012 mg/Actuat Inhalant Powder
| NDC: | 0085-1401 |
| Labeler: | Merck Sharp & Dohme Corp. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Foradil Foradil |
| Dosage Form: | Respiratory (Inhalation) Capsule |
| Application #: | NDA020831 |
| Rev. Date: |
Appearance:
| Markings: | CG;FXF |
| Shapes: |
Capsule |
| Colors: |
 White White |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
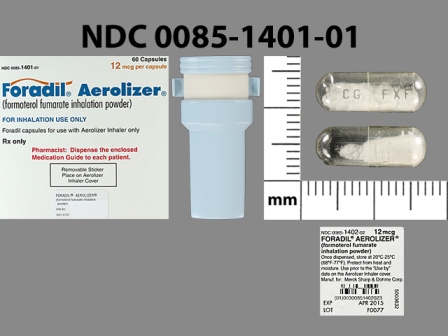
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0085-1401-01: 60 CAPSULE IN 1 BOX, UNIT‑DOSE (0085‑1401‑01)
Active Ingredients:
- Formoterol Fumarate
Dosage Strength:
- 12 ug/1
Inactive Ingredients:
- Lactose
Pharmaceutical Classes:
- Adrenergic beta2-Agonists [MoA]
- beta2-Adrenergic Agonist [EPC]
Related Products:
Based on records with the same trade name.- 0085-1402 Foradil 0.012 mg/Actuat Inhalant Powder by Merck Sharp & Dohme Corp.
- 54868-4972 Foradil 0.012 mg/Actuat Inhalant Powder by Physicians Total Care, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0085-1388Next: 0085-1402 >
Related Discussions:
formoterol 12mcg + budesonide 0.5mg
My husband has copd and is sleepy all the time. Can formoterol make him sleepy? He takes other meds as well but can'... 2 replies
My husband has copd and is sleepy all the time. Can formoterol make him sleepy? He takes other meds as well but can'... 2 replies
formoterol fumarate 6mcg and budesonide 200mcg inhaler for asthma side effects 2times daily and necessity arises ipratropium inhaler 20mcg as required
For the past 4yrs I am taking this medications with other medications tell me the side effects. If cold or allergy affec... 1 reply
For the past 4yrs I am taking this medications with other medications tell me the side effects. If cold or allergy affec... 1 reply
budesonide formoterol
inhaler...
inhaler...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.