0006-0726 : Zocor 5 mg Oral Tablet
| NDC: | 0006-0726 |
| Labeler: | Merck Sharp & Dohme Corp. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Zocor Zocor |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA019766 |
| Rev. Date: |
Appearance:
| Markings: | MSD;726;ZOCOR;5 OR MSD;726;ZOCOR |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
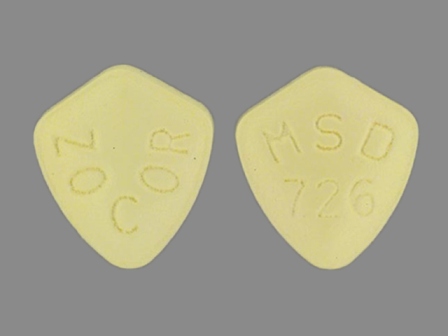
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0006-0726-31: 30 TABLET, FILM COATED IN 1 BOTTLE (0006‑0726‑31)
Active Ingredients:
- Simvastatin
Dosage Strength:
- 5 mg
Inactive Ingredients:
- Ascorbic Acid
- Butylated Hydroxyanisole
- Citric Acid Monohydrate
- Hydroxypropyl Cellulose (Type H)
- Hypromellose 2910 (6 Mpa.s)
- Ferric Oxide Yellow
- Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Starch, Corn
- Talc
- Titanium Dioxide
Pharmaceutical Classes:
- HMG-CoA Reductase Inhibitor [EPC]
- Hydroxymethylglutaryl-CoA Reductase Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0006-0543 Zocor 80 mg Oral Tablet by Merck Sharp & Dohme Corp.
- 0006-0735 Zocor 10 mg Oral Tablet by Merck Sharp & Dohme Corp.
- 0006-0740 Zocor 20 mg Oral Tablet by Merck Sharp & Dohme Corp.
- 0006-0749 Zocor 40 mg Oral Tablet by Merck Sharp & Dohme Corp.
- 49999-306 Zocor 20 mg Oral Tablet by Lake Erie Medical Surgical & Supply Dba Quality Care Products LLC
- 78206-179 Zocor 80 mg Oral Tablet, Film Coated by Organon LLC
- 78206-180 Zocor 10 mg Oral Tablet, Film Coated by Organon LLC
- 78206-181 Zocor 20 mg Oral Tablet, Film Coated by Organon LLC
- 78206-182 Zocor 40 mg Oral Tablet, Film Coated by Organon LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0006-0717Next: 0006-0731 >
Related Discussions:
Simvastatin Tablet Description
I have been taking simvastatin 20MG for sometime now and it was a tan round shaped tablet. I just start a new bottle ref... 15 replies
I have been taking simvastatin 20MG for sometime now and it was a tan round shaped tablet. I just start a new bottle ref... 15 replies
simvastatin 40mg side effects
I have been having a problem with really sore breasts. Could this be a side effect? ## Simvastatin (40 mg). Side effects... 11 replies
I have been having a problem with really sore breasts. Could this be a side effect? ## Simvastatin (40 mg). Side effects... 11 replies
simvastatin 20mg different shape and colour
I have been taking simvastin 20mg for over a year now and it has always been an eliptical tablet with the number 20 impr... 7 replies
I have been taking simvastin 20mg for over a year now and it has always been an eliptical tablet with the number 20 impr... 7 replies
Simvastatin and CoQ10
I was recently told that simvastatin depletes CoQ10, leading to the "weakness" side effect. Anyone heard this? #... 3 replies
I was recently told that simvastatin depletes CoQ10, leading to the "weakness" side effect. Anyone heard this? #... 3 replies
Simvastatin tab and kidney problems
I have been on this medication for five years or longer now. My doctor has stopped it due to tests that came back with n... 3 replies
I have been on this medication for five years or longer now. My doctor has stopped it due to tests that came back with n... 3 replies
Simvastatin and frequent urinating
Hi I'm a 55 year old male and I have been on simvastatin for about 8 years now (40). It has worked wonders with my c... 2 replies
Hi I'm a 55 year old male and I have been on simvastatin for about 8 years now (40). It has worked wonders with my c... 2 replies
simvastatin tabs
I have been taking simvastatin tabs for 2 months now, choslerol count is good but my trigleride level has increased from... 2 replies
I have been taking simvastatin tabs for 2 months now, choslerol count is good but my trigleride level has increased from... 2 replies
simvastatin what colour and shape is tablet
The tablet is white with no markings in shape of a flower ## Then it is not a legal U.S. tablet of Simvastatin, they all... 2 replies
The tablet is white with no markings in shape of a flower ## Then it is not a legal U.S. tablet of Simvastatin, they all... 2 replies
Simvastatin Manufacturer Lupin Is It Gluten Free
Simvastatin 10mg manufacturer Lupin Pharm is it gluten free? ## Hi Pereaone, From what I was able to gather, gluten is n... 2 replies
Simvastatin 10mg manufacturer Lupin Pharm is it gluten free? ## Hi Pereaone, From what I was able to gather, gluten is n... 2 replies
simvastatin 40mg tab
cholesterol ## I need to have the white tablet 20mg as the orange gives chronic headaches and my friend takes 40mg no ph... 2 replies
cholesterol ## I need to have the white tablet 20mg as the orange gives chronic headaches and my friend takes 40mg no ph... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.